
Mix-up of samples in blood gas testing
Avoid mixing up blood gas samples and get the correct results for your patient
Consequences of the mix-up of blood gas samples
Accurate specimen labeling is critical. Mixing up samples can occur due to healthcare professionals failing to match patient identifiers with the correct order, failing to label a sample immediately after blood collection or transcription errors from manual data entry. [1-3]
These errors may have serious consequences. The mix-up of blood gas samples can lead to misdiagnosis, failure to provide proper care, lost billing opportunities or the requirement of resampling. Mixing up blood gas samples can lead to results of no clinical value or worse, adverse patient outcomes. [1] [3]
Avoiding the mix-up of blood gas samples, a priority
The Clinical and Laboratory Standards Institute (CLSI) recommends labeling blood gas samples with the patient’s full name and second identifier. [4]
Mixing up blood gas samples is a growing concern.
In January 2022, the U.S. Joint Commission on Accreditation of Healthcare Organizations (JCAHO) released their annual National Patient Safety Goals, the purpose of which is to improve patient safety.
The JCAHO listed “improve accuracy of patient identification” as their number one National Patient Safety Goal for 2022 in the Hospital Program. [5]
1st Automatic, integrated with safePICO syringes
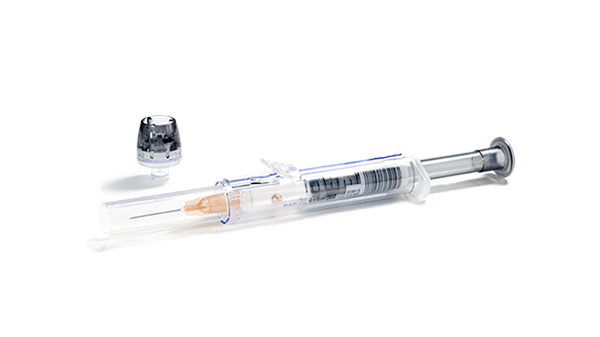
Identify patients correctly with 1st Automatic and minimize the risk of patient mix-up. This automated data registration system connects the caregiver, the sample, and the patient. 1st Automatic works with the safePICO syringe for correct, automatic linkage between the blood gas sample you collect and the patient.
The safePICO syringe is designed to reduce the risk of preanalytical errors. In addition to mixing up blood gas samples, safePICO can help to remove air bubbles safely from the sample and to reduce the risk of a clotted blood sample, hemolysis due to mixing, or needlestick injuries.

References
2. Simundic AM et al. Compliance of blood sampling procedures with the CLSI H3-A6 guidelines: An observational study by the EFLM working group for the preanalytical phase (WG-PRE). Clin Chem Lab Med. 2015 Aug; 53(9); 1321-1331.
3. Mays JA, Mathias PC. Measuring the rate of manual transcription error in outpatient point-of-care testing. J Am Med Inform Assoc. 2019 Jan 10; 26(3) 269-272.
4. CLSI. Blood Gas and pH Analysis and Related Measurements; Approved Guideline—Second Edition. CLSI document C46-A2 [ISBN 1-56238-694-8)
5. The Joint Commission. Hospital: 2022 National Patient Safety Goals. https://www.jointcommission.org/-/media/tjc/documents/standards/national-patient-safety-goals/2022/npsg_chapter_hap_jan2022.pdf. Accessed October 2022.
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.

